Molnupiravir
When it enters the cell it is converted into RNA-like building blocks. Molnupiravir demonstrated activity in preclinical models of SARS-CoV-2 SARS-CoV-1 and MERS including prophylaxis cure and transmission prevention.
Molnupiravir an Oral Antiviral Treatment for COVID-19.

Molnupiravir. Molnupiravir is the first oral direct-acting antiviral shown to be highly effective at reducing nasopharyngeal SARS-CoV-2 infectious virus and viral RNA and has a favorable safety and tolerability profile. Molnupiravir a wide-spectrum antiviral that is currently in phase 23 clinical trials for the treatment of COVID-19 is proposed to inhibit viral replication by a mechanism known as lethal. Timelines as to when to use molnupiravir and the patients vaccination status are confounding factors in gathering efficacy evidence in the trial and in practice if it garners regulatory support.
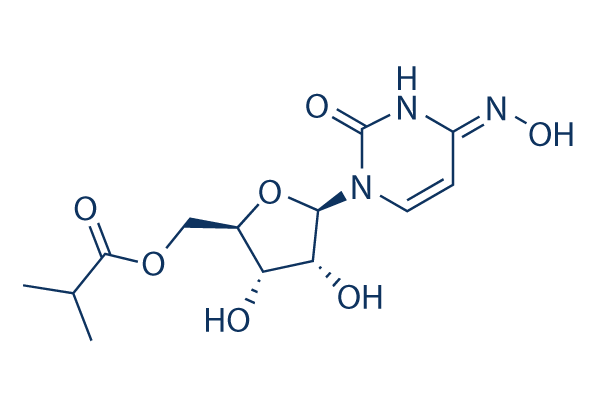
Merck on Friday announced its anti-viral drug has shown compelling results in clinical trials halving the risk of hospitalisation or death for patients with mild or moderate cases of Covid. Molnupiravir drug is an orally effective prodrug of the manufactured nucleoside evolved N4-hydroxycytidine. In the first phase the.
Molnupiravir an oral antiviral treatment for COVID-19. A novel coronavirus originally identified in Wuhan City China was reported to the World Health Organization on 31 December 2019 and the associated disease has subsequently become a worldwide pandemic. In the first phase the.
Molnupiravir tricks the coronavirus into using the drug to try to replicate the viruss genetic material. Molnupiravir FDA Approval Status. In March 2021 the companies reported preliminary results from a Phase IIa trial of molnupiravir for Covid-19.
Government will procure approximately 17 million courses of an investigational antiviral treatment molnupiravir MK-4482 for COVID-19 from Merck pending emergency use authorization EUA or approval from the US. When it enters the cell it is converted into RNA-like building blocks. If authorized by the Food and Drug Administration FDA the drug molnupiravir could be the first oral antiviral treatment for patients with COVID-19.
Background Easily distributed oral antivirals are urgently needed to treat coronavirus disease-2019 COVID-19 prevent progression to severe illness and block transmission of severe acute respiratory syndrome coronavirus 2 SARS-CoV-2. Molnupiravir is an orally available antiviral drug candidate currently in phase III trials for the treatment of patients with COVID-19. As of June 25 2021 SARS-CoV-2.
We report the results of a Phase 2a trial evaluating the safety tolerability and antiviral efficacy of molnupiravir in the treatment of COVID-19. Molnupiravir is an orally available drug which becomes activated through metabolization in the body. Molnupiravir was generally well tolerated with similar numbers of adverse events across all groups.
Here we establish the molecular mech. Molnupiravir MK-4482EIDD-2801 is an investigational orally administered form of a potent ribonucleoside analog that inhibits the replication of SARS-CoV-2 the causative agent of COVID-19. Molnupiravir has been shown to be active in several preclinical models of SARS-CoV-2 including for prophylaxis treatment and prevention of transmission.
Molnupiravir increases the frequency of viral RNA mutations. It prompts errors in the viral RNA grouping stopping the viral replication lessening the contamination and restricting infection transmission during the viral RNA replication. Molnupiravir originally created by researchers at Emory University in Atlanta is given as four pills taken twice a day for five days.
Merck Molnupiravir EIDD-2801MK-4482 is an investigational orally bioavailable form of a potent ribonucleoside analog in development for the treatment of COVID-19. Molnupiravir is also being evaluated for whether it can help prevent transmission of virus or as prophylaxis in MOVe-AHEAD a global multicentre randomised double-blind placebo-controlled. Food and Drug Administration FDA.
The Biden Administration today announced that the US. Given were still averaging 122 deaths a day from COVID in the UK despite high levels of vaccination a. Last updated by Judith Stewart BPharm on July 14 2021.
Molnupiravir is a promising and clever drug but we need more information. Of the participants who received molnupiravir. Molnupiravir has an attractive oral formulation ideal for outpatient use but a lack of long-term data may limit initial rollout to high-risk people.
All you need to know about Mercks COVID-19 oral pill. Molnupiravir increases the frequency of viral RNA mutations and impairs SARS-CoV-2 replication in animal models and in humans. Merck said it planned to seek emergency.
Molnupiravir co-developed with Ridgeback Biotherapeutics is. Molnupiravir is an orally available drug which becomes activated through metabolization in the body. Molnupiravir ska testas i Sverige mot covid-19 - Life Science Sweden.
Molnupiravir is an orally available antiviral drug candidate currently in phase III trials for the treatment of patients with COVID-19. Once that process is underway the drug inserts errors into the genetic code. An effective antiviral therapeutic has since been intensively sought.

Molnupiravir Last Of The Small Molecule Coronavirus Hopes Science Aaas
Lung Center Qmmc Call For Participants In Molnupiravir Trials Vs Covid 19

Anti Viral Pill Molnupiravir Shows Promise Against Covid Other Viruses Youtube

A Daily Pill To Treat Covid Could Be Just Months Away Scientists Say

Molecular Mechanisms Of Corona Drug Candidate Molnupiravir Unraveled Max Planck Gesellschaft